
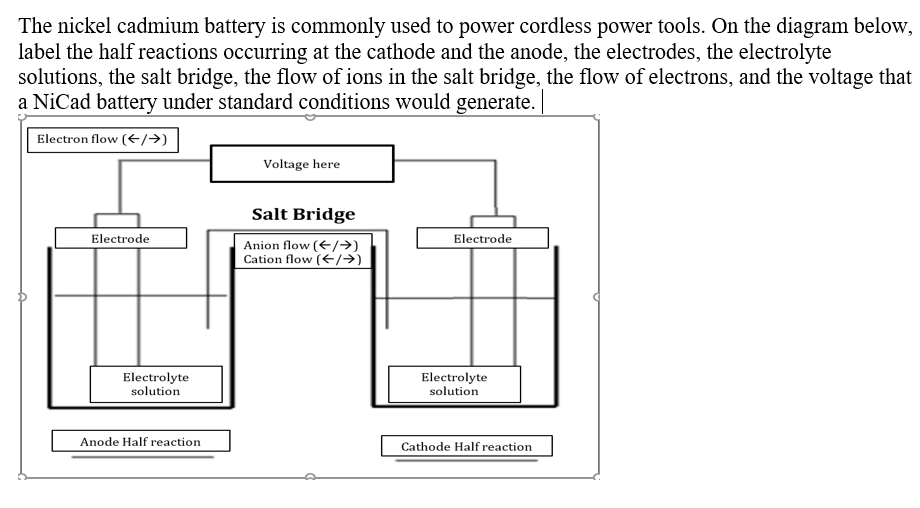
Parts of the electrochemical cell (KEY TERMS):
#Salt bridge battery cathode and anode series
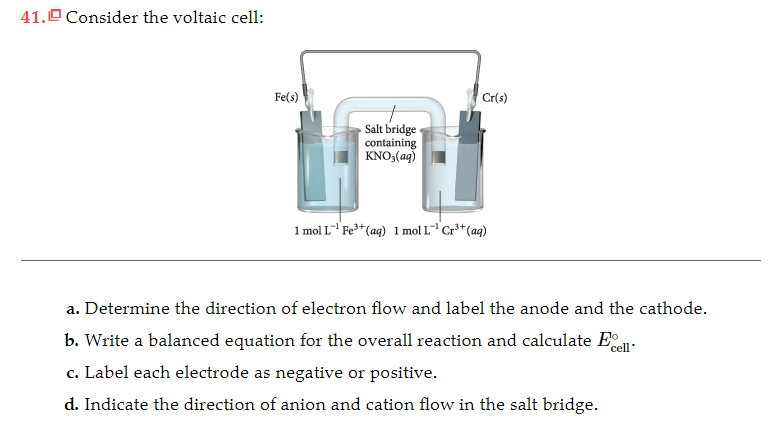
Zn is above Cu in activity series so Zn can displace Cu ionsĬu2+(aq) + 2e- → Cu(s) Reduction (Cathode).Other half cell consists of a piece of copper in One half cell contains a piece of zinc placed in a One of the first galvanic cells was developed by The half-reactions occur in each of the separate Can harness this electron flow to light a light bulbĪ galvanic (voltaic) cell may be created using Important that redox reactants are not in direct contact, but electrons flow through an electric circuit Converts chemical energy to electrical energy Also called Voltaic Cells or Galvanic Cells A current will continue to flow until the cell reaches equilibrium Batteries operate by allowing electrons to spontaneously flow (electrical current) from the Lehninger Principles of Biochemistry (Albert Lehninger Michael Cox David L.Yates Teresa Bereznicki-korol Trevor Clarke) Child Psychology (Alastair Younger Scott A.Behavioral Neuroscience (Stéphane Gaskin).Bioethics: Principles, Issues, and Cases (Lewis Vaughn).
Organizational Behaviour (Nancy Langton Stephen P.Vitale Joseph Giglierano Waldemar Pfoertsch) Business-To-Business Marketing (Robert P.Cognitive Psychology (Robert Solso Otto H.Instructor's Resource CD to Accompany BUSN, Canadian Edition Kelly, McGowen, MacKenzie, Snow (Herb Mackenzie, Kim Snow, Marce Kelly, Jim Mcgowen).Psychology : Themes and Variations (Wayne Weiten).Introduction to Corporate Finance WileyPLUS Next Gen Card (Laurence Booth).Sílabo de Emprendimiento para el Desarrollo Sostenible.Happiness - Copy - this is 302 psychology paper notes, research n Wrap-up - this is 302 psychology paper notes, researchpsy

#Salt bridge battery cathode and anode free
Paris Gennaro- Gizmo Free Fall SE - Google Docs.CCNAv 7 System Test Course (Version 1.1) – System Test Exam Answers.Accounting Principles Solution Chapter (13).Customer Service Skills for Success 7Th Edition By Robert Lucas Test Bank.Internal Competition - A curse for Team Performance.Chapter 1 Notes - Summary Psychology : Themes and Variations.Biol 1902 - if u got biol 1902 with mike runtz these notes got ur back.Lecture notes, lectures 10 - Sociolinguistics.Lecture Notes Income Taxation Canada Winter.Chapter 1-Thinking Critically with Psychological Science.Summary Auditing a Practical Approach - Chapter 4-6.Essential Communication Skills (COMM 19999).Introductory Pharmacology and Therapeutics (Pharmacology 2060A/B).Adult Health and Health Alterations (Nurs 400).Introduction to Probability and Statistics (STAT 1201).Medical Microbiology for Health Care Professionals (Mmi133).Fundamentals Of Cell Biology (BIOL 200).Introduction to UNIX/Linux and the Internet (ULI 101).Ancient Roots of Medical Terminology (Classics 2Mt3).Analyse, écriture et argumentation (FRA1710).If you measure the voltage under load, it will also be affect by the nature of the salt bridge. If ion transport along the salt bridge is the rate-determining step, it will affect the current (current is movement of charge per time). The voltage and the current depend on what load you attach to the voltaic cell, and properties of the cell itself. A similar (but reversed) situation is found in the cathodic cell, where $\ce$ in making the salt bridge have any effects on voltage/current output of the cell? why? In order to maintain neutrality, the negatively charged ions in the salt bridge will migrate into the anodic half cell. The electrons move through the wire (and your device, which I haven't included in the diagram), leaving the unbalanced positive charge in this vessel. The oxidation reaction that occurs at the anode generates electrons and positively charged ions. The electrons flow from the anode to the cathode. The purpose of a salt bridge is not to move electrons from the electrolyte, rather it's to maintain charge balance because the electrons are moving from one-half cell to the other. There's another question related to salt bridges on this site.


 0 kommentar(er)
0 kommentar(er)
